- Get link
- X
- Other Apps
A type of immunotherapy called CAR T-cell therapy is now an option for some people with multiple myeloma. CAR T cells however are unlike checkpoint inhibitorsor any other approved cancer therapy.
 Post Fda Approval Car T Therapy Key Facts
Post Fda Approval Car T Therapy Key Facts
Introduction Classifications FDA Approved Drugs Side Effects.
Car t therapy fda. Idecabtagene vicleucel also called ide-cel and. Tisagenlecleucel Kymriah is approved in order to treat relapsedrefractory B-cell precursor acute lymphoblastic leukemia ALL while axicabtagene ciloleucel Yescarta is approved in order to treat relapsedrefractory diffuse large B-cell lymphoma DLBCL. Operation chemotherapy and radiotherapy.
FDA Approves First CAR T-Cell Therapy The evolution of CAR T-Cell Therapy. By binding to the BCMA protein the CAR T cell therapy can kill cancerous myeloma cells. UPMC Hillman Cancer Center is the first in western Pennsylvania to provide TECARTUS TM brexucabtagene.
CAR T Therapy. Having the first CAR-T cell therapy approved in myeloma is wonderful news for the patient community. The FDA approved the therapeutic on the basis of a single-arm trial of lisocabtagene maraleucel preceded.
FDA-approved CAR T-cell Therapies ABECMA idecabtagene vicleucel. The FDA has approved Bristol Myers Squibbs idecabtagene vicleucel for multiple myeloma a first green light for a BCMA-targeted CAR-T cell therapy. Cancer treatment has historically relied on what are called the three pillars of oncology.
5 In addition because CART cells are cellbased gene therapies three of the suites of six draft gene therapy guidance documents published in May 2018 for comment are relevant to their production. May 19 2020 - FDA recently issued a Refusal to File letter to Bristol Myers Squibb BMS and Bluebird Bio regarding the Biologics License Application BLA for idecabtagene vicleucel a chimeric antigen receptor CAR T cell immunotherapy for patients with multiple myeloma. UPMC Hillman Cancer Center was one of the first in the United States certified to.
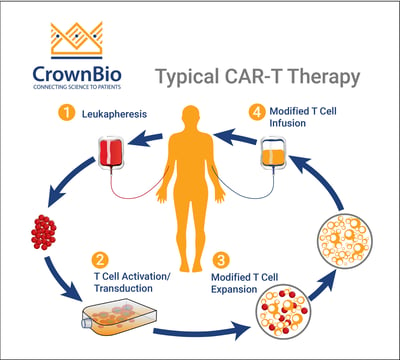
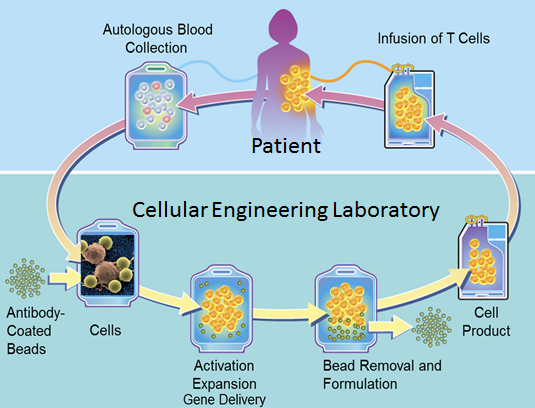
FDA approves BMS blood cancer CAR T therapy after delay The FDAs approval of Breyanzi came a year after the start of the review The US Food and Drug Administration FDA has finally approved Bristol Myers Squibbs BMS CAR T-cell therapy Breyanzi previously known as liso-cel after delaying the decision in November 2020. The FDA today also expanded the approval of Actemra tocilizumab to treat CAR T-cell-induced severe or life-threatening CRS in patients 2 years of age or older. CAR T Therapy is a patient-specific treatment in which T cells are isolated from the patient by apheresis and cultured using cytokines to actively proliferate and increase in number.
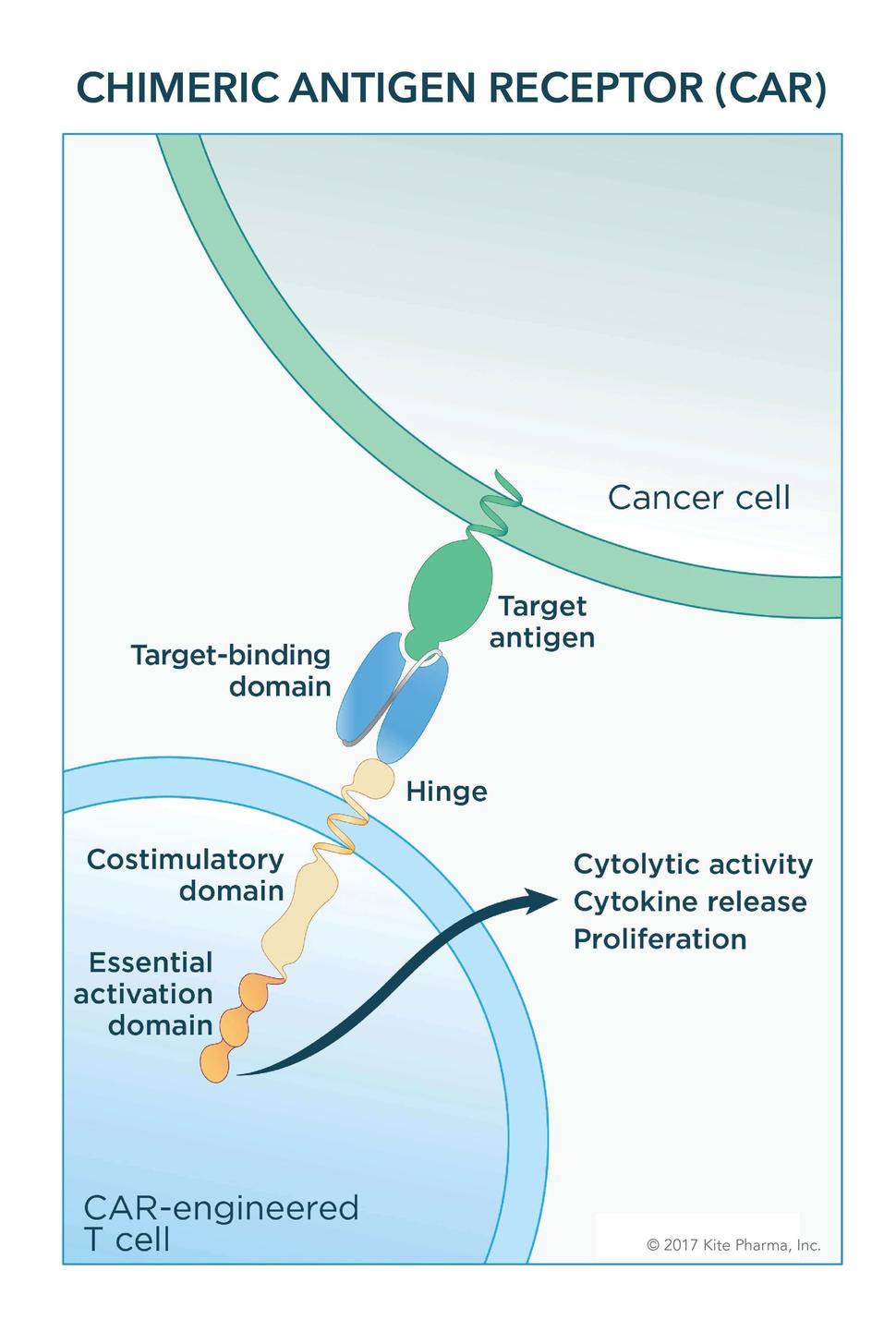
Lisocabtagene maraleucel is a CD19-directed chimeric antigen receptor CAR-T cell therapy. Expanded T cells are transduced with gene encoding CAR Chimeric Antigen Receptor. The first two FDA-approved CAR-T therapies both target CD19 antigen and found on many types of B-cell cancers.
On March 26 the Food and Drug Administration FDA approved idecabtagene vicleucel Abecma for people with multiple myeloma that has not responded to or has returned after at least four different prior cancer treatments. As an anti-BCMA CAR-T cell therapy this drug recognises and binds to BCMA a protein that is nearly universally expressed on cancer cells in myeloma. Yescarta a chimeric antigen receptor CAR T cell therapy is the second gene therapy approved by the FDA and the first for certain types of non-Hodgkin lymphoma NHL.
Decades of Research Lead to CAR T-Cell Therapy Approvals The most widely used type of immunotherapy is a class of drugs known as immune checkpoint inhibitors which have been approved by FDA for the treatment of a variety of solid and blood cancers. In clinical trials in patients. As we look to bring the hope of survival to more patients in need todays FDA decision represents a real step forward in our commitment in hematologic malignancies said Christi Shaw Chief Executive Officer of Kite in a press release.
It is the first CAR T-cell therapy approved for FL. In November 2017 to better clarify the regulatory landscape for regenerative medicine products including CART cells CBER issued a suite of regenerative medicine guidance documents.
 Fda Approves First Car T Cell Therapy For Pediatric Acute Lymphoblastic Leukemia Nih Director S Blog
Fda Approves First Car T Cell Therapy For Pediatric Acute Lymphoblastic Leukemia Nih Director S Blog
 A Cure For Cancer How Car T Cell Therapy Is Revolutionizing Oncology
A Cure For Cancer How Car T Cell Therapy Is Revolutionizing Oncology
 Fda Approves Second Car T Cell Therapy National Cancer Institute
Fda Approves Second Car T Cell Therapy National Cancer Institute
 Car T Therapy Ohc Oncology Hematology Care
Car T Therapy Ohc Oncology Hematology Care
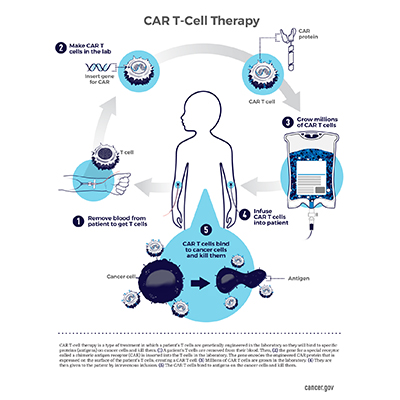 Fda Grants Breakthrough Therapy Designation For New Car T Cell Therapy For B Cell Acute Lymphoblastic Leukemia Center For Cancer Research National Cancer Institute
Fda Grants Breakthrough Therapy Designation For New Car T Cell Therapy For B Cell Acute Lymphoblastic Leukemia Center For Cancer Research National Cancer Institute
Adopt Car T Cell Therapy February 2019 Oncology Pharmacy Purchasing Products Magazine
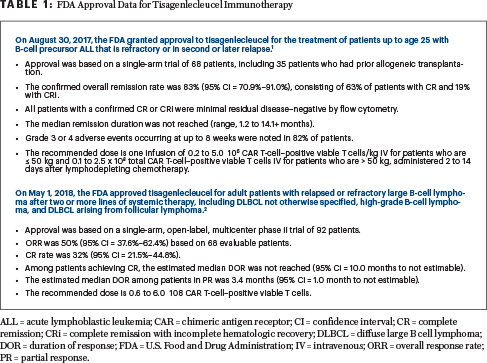 Update On Fda Approved Car T Cell Products Tisagenlecleucel The Asco Post
Update On Fda Approved Car T Cell Products Tisagenlecleucel The Asco Post
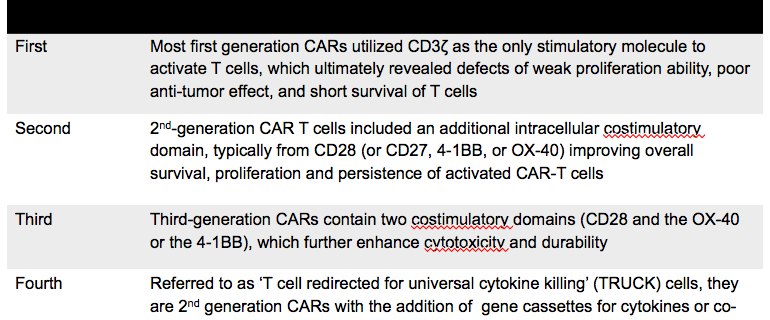 Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
 Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
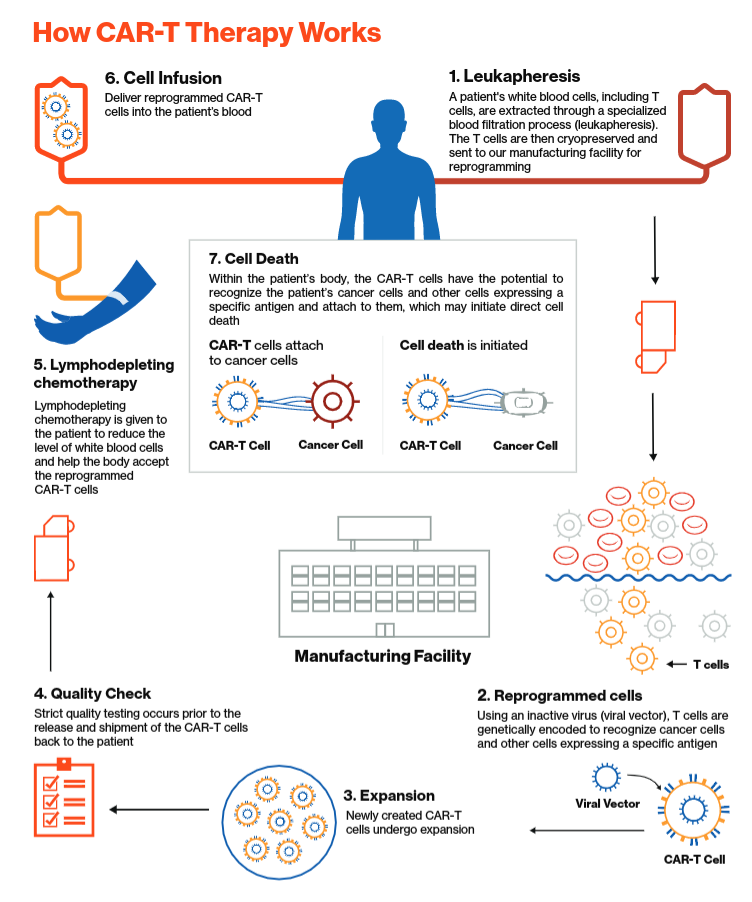 The Fda Approves A Second Car T Therapy Cheaper Than Novartis
The Fda Approves A Second Car T Therapy Cheaper Than Novartis
 Update On Fda Approved Car T Cell Gene Therapy For B Cell Lymphomas The Asco Post
Update On Fda Approved Car T Cell Gene Therapy For B Cell Lymphomas The Asco Post
 Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
 Historical Timeline Of The Clinical Translation Of Chimeric Antigen Download Scientific Diagram
Historical Timeline Of The Clinical Translation Of Chimeric Antigen Download Scientific Diagram
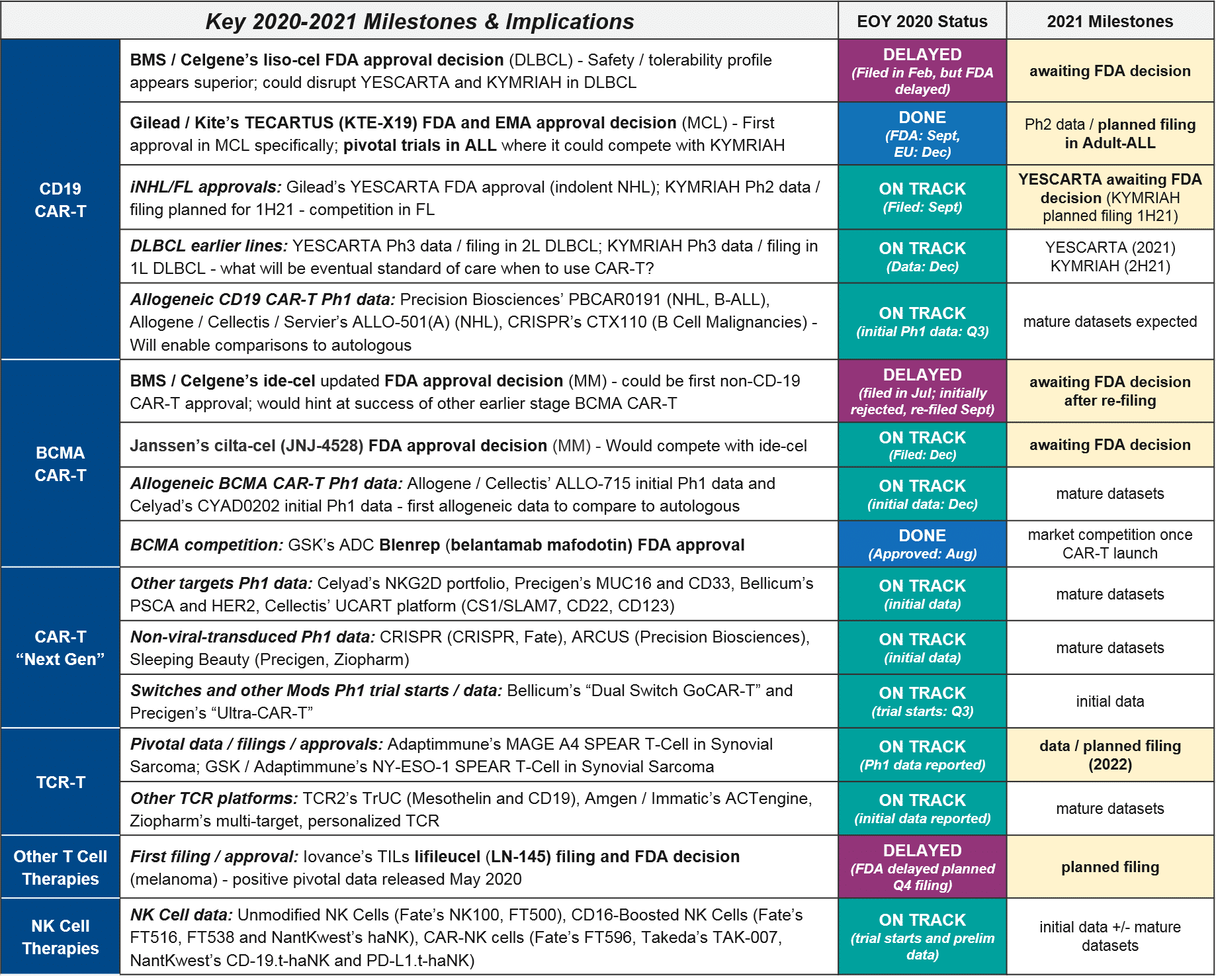
 2021 Outlook For Cell Therapies In Oncology Blue Matter Consulting
2021 Outlook For Cell Therapies In Oncology Blue Matter Consulting
Comments
Post a Comment