- Get link
- X
- Other Apps
There is a huge need to generate more data for all of the drugs that pregnant women need to take Christina Chambers PhD MPH Lead Investigator MotherToBaby Pregnancy Studies. X US FDA pregnancy category.
Women who become pregnant while taking AUBAGIO may enroll in the AUBAGIO pregnancy registry by calling 1-800-745-4447 option 2.

Aubagio pregnancy category. Teriflunomide Pregnancy and Breastfeeding Warnings. Last updated on Dec 1 2020. Breast-feeding women see section 46.
Aubagio also carries a boxed warning on the risk of teratogenicity. Teriflunomide is a pregnancy category X drug based on animal studies demonstrating fetal harm. In clinical trials MRI scans showed people taking Aubagio had fewer smaller or no new areas of active MS lesions.
For the full list of restrictions see the package leaflet. If pregnancy occurs during teriflunomide treatment April 2013 Updated version may be found at wwwpbmvagov or vawwpbmvagov 2. Pregnant women or women of childbearing potential who are not using reliable contraception during treatment with teriflunomide and thereafter as long as its plasma levels are above 002 mgl see section 46.
Aubagio has a pregnancy category X. Aubagio may increase the risk of birth defects. The FDA categorizes medications based on safety for use during pregnancy.
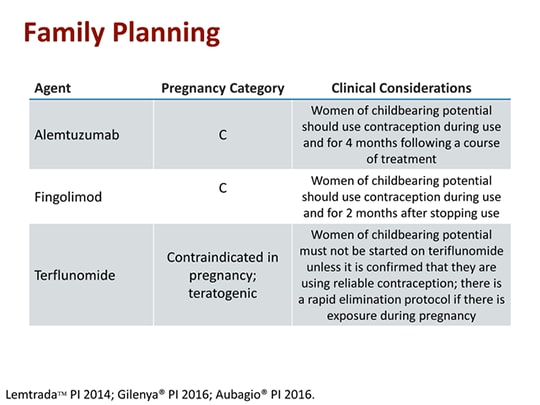
Aubagio may cause harm to your unborn baby. Therefore it is contraindicated in pregnant women or women of childbearing potential who are not using reliable contraception. Read more about Aubagio.
Teriflunomide is approved for treatment of relapsing forms of. Aubagio must also not be used in pregnant women or during breast-feeding. Aubagio may also slow down the build-up of disability associated with MS.
Taking Aubagio with certain birth control pills may increase your bodys levels of the hormones in the. Women who can become pregnant must not take Aubagio without using reliable contraceptive measures. Aubagio is Pregnancy Category X as designated by the FDA meaning that it may cause major birth defects if used during pregnancy.
This medication falls into category X. Oral contraceptives birth control pills are medications that help prevent pregnancy. Teriflunomide is also known as.
Teriflunomide is eliminated slowly from the plasma-it takes an average of 8 months or up to 2 years to reach plasma concentrations. The International Teriflunomide Pregnancy Exposure Registry will compare rates of birth defects congenital malformations fetal deaths termination due to fetal abnormality in teriflunomide-exposed pregnant women with those reported by the European Surveillance of Congenital Anomalies EUROCAT. Pregnancy must be excluded before start of treatment see section 46.
Pregnancy must be excluded before start of treatment see section 46. Pregnancy must be avoided during Aubagio treatment or. Aubagio is moderately effective category 11 DMD.
However the subsequent TENERE head-to-head comparison trial reported that although permanent discontinuations were substantially less. MotherToBaby is currently enrolling pregnant women in a study examining the use of Aubagio teriflunomide to treat multiple sclerosis during pregnancy. Do not take it if.
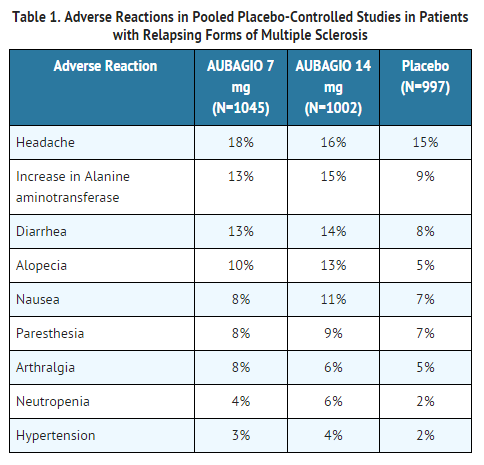
The study was completed in July 2010. Pregnant women or women of childbearing potential who are not using reliable contraception during treatment with teriflunomide and thereafter as long as its plasma levels are above 002 mgl see section 46. In clinical trials people taking Aubagio had about 30 fewer relapses than people taking placebo.
2-year results were positive. For this reason Aubagio is labeled as Pregnancy Category X which means women of childbearing age must have a negative pregnancy test before starting the. Teriflunomide was investigated in the Phase III clinical trial TEMSO as a medication for multiple sclerosis.
You must not become pregnant while taking Aubagio and for a certain period of time after stopping Aubagio. Aubagio is contraindicated in pregnant women or women of childbearing potential who are not using reliable contraception. Pregnancy must be avoided during teriflunomide treatment.
AU TGA pregnancy category. Five categories - A B C D and X are used to classify the possible risks to an unborn baby when a medication is taken during pregnancy. Do not take Aubagio if you are pregnant.
Teriflunomide sold under the brand name Aubagio is the active metabolite of leflunomide. Women of childbearing potential must use reliable contraception while taking Aubagio. Sanofi Genzymes multiple sclerosis therapy Aubagio teriflunomide does not appear to cause birth defects in humans as it does in laboratory animals researchers concluded after.









Comments
Post a Comment